A balance is required
Co-morbidity is a serious concern in clinical trials, introducing complexity that significantly impacts the validity, safety, and general application of the study’s findings. Cohorts with multiple conditions make trials harder to validate efficacy but the results, if positive, are more applicable to a general population.
This is particularly true when studying conditions that present within older populations or rare disease. With these cohorts, where co-morbidity is more common or treatments are simply to alleviate symptoms with a genetic aspect, these trialists may respond differently to the treatment being investigated.
Pre-existing treatment regimens can lead to adverse drug reactions. The genetics of rare disease patients can alter drug metabolism. All this leads to increased complexity in trials.
Clinical trial organisations must balance this complexity with applicability of results found.

Why compromise?
To reduce complexity, most clinical trials have strict exclusion criteria for their cohorts. This may sacrifice the resulting applicability of the treatment if found to be an effective therapy, but rendering it effective only in limited scenarios.
Trialist exclusion can be thought of reducing your cohort horizontally to a point where complexity is manageable; but is does not have to be so.
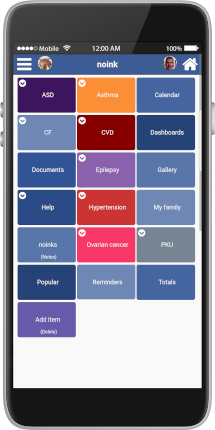
noink is capable of handling co-morbidity by design, allowing life science organisations to include trialists who would otherwise be excluded. As each real-world datapoint is recorded in a structured manner, accounting for any morbidity, any inclusion or exclusion of data can be processed in real time in the context of a particular trialist.
noink has been designed to handle the RWD recording needs for any and all conditions. We have abstracted data capture from underlying morbidity. Conditions can be configured immediately for any particular trial giving rapid scale-up of of your research.
There’s more
Selection issues aside, the co-morbidity functionality of noink gives potential trialists the ability to record off-study data for themselves from any of our available conditions. Helping patients use technology to improve their health.
This added utility to assist any patient’ health journey, irrespective of any trial, increases the engagement of trialists. Increased engagement has been shown to increase cohort retention.
Increased patient utility breaks the transactional nature of current trials. noink allows life science organisations to maintain a larger cohort for any study without compromising data quality, while also giving patients more utility in managing their health. noink allows the running of larger trials with a more engaged cohort.

